Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O(
O( He,
He, )
) F reaction; Identification of the low-energy "super" -Gamow-Teller state
F reaction; Identification of the low-energy "super" -Gamow-Teller stateFujita, Hirohiko*; Fujita, Yoshitaka*; Utsuno, Yutaka; Yoshida, Kenichi*; Adachi, Tatsuya*; Algora, A.*; Csatl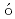 s, M.*; Deaven, J. M.*; Estevez-Aguado, E.*; Guess, C. J.*; et al.
s, M.*; Deaven, J. M.*; Estevez-Aguado, E.*; Guess, C. J.*; et al.
Physical Review C, 100(3), p.034618_1 - 034618_13, 2019/09
Times Cited Count:12 Percentile:76.6(Physics, Nuclear)no abstracts in English
 CsI +
CsI +  Cs
Cs 
 Cs + I
Cs + I Cs
CsKobayashi, Takanori*; Matsuoka, Leo*; Yokoyama, Keiichi
Computational and Theoretical Chemistry, 1150, p.40 - 48, 2019/02
Times Cited Count:1 Percentile:1.99(Chemistry, Physical)One of important research targets in the development of cesium isotope separation system is design of recovery process of cesium atom. Relevant to this research target, the reaction cross section and reaction rate constant of a cesium exchange reaction through collision of the cesium iodide molecules with cesium atoms are calculated by a quasi-classical trajectory calculation based on a potential energy surface obtained by quantum chemistry calculations. Consequently, the rate constant is calculated to be 3.6  10
10 cm
cm molecule
molecule s
s , as large as collision rate in the present condition. In addition, slightly positive temperature dependence is observed in the rate constant. This behavior is explained with the long-range attractive force and effect of subsequent dissociation process.
, as large as collision rate in the present condition. In addition, slightly positive temperature dependence is observed in the rate constant. This behavior is explained with the long-range attractive force and effect of subsequent dissociation process.
 CsI (v = 0, j = 0) +
CsI (v = 0, j = 0) +  Cs
Cs 
 Cs + I
Cs + I Cs
CsKobayashi, Takanori*; Matsuoka, Leo*; Yokoyama, Keiichi
Nihon Enerugi Gakkai-Shi, 96(10), p.441 - 444, 2017/10
To investigate the reaction cross section of the cesium exchange reaction of  CsI (v = 0, j = 0) +
CsI (v = 0, j = 0) +  Cs
Cs 
 Cs + I
Cs + I Cs, we performed quasi-classical trajectory calculations on the potential energy surface calculated by the ab initio molecular orbital theory. The potential energy surface shows that intermediate Cs
Cs, we performed quasi-classical trajectory calculations on the potential energy surface calculated by the ab initio molecular orbital theory. The potential energy surface shows that intermediate Cs I is formed without entrance barrier and has two equivalent Cs-I bonds. The reaction cross sections decrease monotonically with increasing collision energy. The rate constant k (v = 0, j = 0) was estimated to be about 3
I is formed without entrance barrier and has two equivalent Cs-I bonds. The reaction cross sections decrease monotonically with increasing collision energy. The rate constant k (v = 0, j = 0) was estimated to be about 3 10
10 cm
cm molecule
molecule s
s at temperatures ranging from 500 to 1200K and a slight negative temperature dependence was observed.
at temperatures ranging from 500 to 1200K and a slight negative temperature dependence was observed.
 Cs + ICs' (Contract research)
Cs + ICs' (Contract research)Kobayashi, Takanori; Hashimoto, Masashi; Yokoyama, Keiichi
JAEA-Research 2015-014, 7 Pages, 2015/12
To discuss the exchange reaction of Cs isotope by CsI + Cs' Cs + ICs', the structure and chemical properties of Cs
Cs + ICs', the structure and chemical properties of Cs I intermediate and potential energy surface are calculated using M06/def2-TZVPPD density functional calculation. The calculation shows that the reaction to the intermediate has no barrier and the two Cs-I bonds of Cs
I intermediate and potential energy surface are calculated using M06/def2-TZVPPD density functional calculation. The calculation shows that the reaction to the intermediate has no barrier and the two Cs-I bonds of Cs I are chemically equivalent. Thus, the collision of CsI + Cs' results in Cs exchange with the high probability.
I are chemically equivalent. Thus, the collision of CsI + Cs' results in Cs exchange with the high probability.
 with surface hydroxyl groups in lithium silicates
with surface hydroxyl groups in lithium silicatesNakazawa, Tetsuya; Yokoyama, Keiichi; Grismanovs, V.*; Katano, Yoshio*; Jitsukawa, Shiro
Journal of Nuclear Materials, 307-311(Part2), p.1436 - 1440, 2002/12
Times Cited Count:1 Percentile:10.13(Materials Science, Multidisciplinary)no abstracts in English
Nakazawa, Tetsuya; Yokoyama, Keiichi; Grismanovs, V.*; Katano, Yoshio*; Jitsukawa, Shiro
Journal of Nuclear Materials, 302(2-3), p.165 - 174, 2002/04
Times Cited Count:3 Percentile:23.39(Materials Science, Multidisciplinary)no abstracts in English
 O molecules from surface hydroxyl groups in silicates
O molecules from surface hydroxyl groups in silicatesNakazawa, Tetsuya; Yokoyama, Keiichi; Grismanovs, V.*; Katano, Yoshio*
Journal of Nuclear Materials, 297(1), p.69 - 76, 2001/07
Times Cited Count:9 Percentile:56.04(Materials Science, Multidisciplinary)no abstracts in English
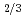 TiO
TiO
Yoshii, Kenji
Journal of Alloys and Compounds, 305(1-2), p.72 - 75, 2000/06
Times Cited Count:13 Percentile:61.56(Chemistry, Physical)no abstracts in English
Sherrill, B. M.*; Akimune, Hidetoshi; Austin, S. M.*; Bazin, D.*; Van den Berg, A. M.*; Berg, G. P. A.*; Caggiano, J.*; Daito, Izuru*; Fujimura, Hisako*; Fujita, Yoshitaka*; et al.
Nuclear Instruments and Methods in Physics Research A, 432(2-3), p.299 - 304, 1999/08
Times Cited Count:39 Percentile:91.81(Instruments & Instrumentation)no abstracts in English
Nishikawa, Masabumi*; *; Kawamura, Yoshinori; Nishi, Masataka
JAERI-Conf 98-006, p.170 - 182, 1998/03
no abstracts in English
 ZrO
ZrO packed bed
packed bedKawamura, Yoshinori; Enoeda, Mikio; Okuno, Kenji
Fusion Engineering and Design, 39-40, p.713 - 721, 1998/00
Times Cited Count:8 Percentile:57.22(Nuclear Science & Technology)no abstracts in English
Nishikawa, Masabumi*; Baba, Atsushi*; *; Kawamura, Yoshinori
Fusion Engineering and Design, 39-40, p.615 - 625, 1998/00
Times Cited Count:14 Percentile:72.98(Nuclear Science & Technology)no abstracts in English
 , He and Li atoms and ions with atoms and molecules,IV
, He and Li atoms and ions with atoms and molecules,IVIto, Rinsuke*; Tabata, Tatsuo*; Shirai, Toshizo; R.A.Phaneuf*
JAERI-Data/Code 96-024, 62 Pages, 1996/07
no abstracts in English
Kawamura, Yoshinori; Nishikawa, Masabumi*; *; Okuno, Kenji
Journal of Nuclear Materials, 230, p.287 - 294, 1996/00
Times Cited Count:43 Percentile:94.6(Materials Science, Multidisciplinary)no abstracts in English
Yui, Mikazu; Shibata, Masahiro; Makino, Hitoshi; ; ; Ishiguro, Katsuhiko; Ishikawa, Hirohisa
PNC TN8410 92-162, 140 Pages, 1992/09
None
 O(VOM-21H); Tritium Chemical Form as a Function of Sweep Gas Composition
O(VOM-21H); Tritium Chemical Form as a Function of Sweep Gas Composition; ; Kurasawa, T.; ;
JAERI-M 86-130, 20 Pages, 1986/09
no abstracts in English
Kurasawa, T.; ; ; ; ;
JAERI-M 84-087, 55 Pages, 1984/05
no abstracts in English
Nakashima, Mikio; Tachikawa, Enzo; Saeki, Masakatsu;
Journal of Inorganic and Nuclear Chemistry, 43(2), p.369 - 373, 1981/00
Times Cited Count:8 Percentile:37.89(Chemistry, Inorganic & Nuclear)no abstracts in English
; ; Motoishi, Shoji; ;
JAERI-M 5320, 31 Pages, 1973/07
no abstracts in English